With some of the nation’s most innovative researchers in pediatric cardiology, the Children’s Heart Center is transforming the health and well-being of children with heart disease in our community and around the world.
Doff McElhinney, MD
Director, Clinical and Translational Research Program, Children’s Heart Center at Lucile Packard Children’s Hospital Stanford
What if you could take all the physician notes, all the MRIs, all the x-rays and lab results that patients have accumulated at different doctors’ offices and hospital visits and bring them all together? What insights and patterns might doctors and researchers learn by analyzing large-scale data?
Doff McElhinney, MD, professor of cardiothoracic surgery at Stanford University School of Medicine, is driving progress toward finding out what patient care in that kind of connected world might look like.
As McElhinney explains, electronic patient data and data-gathering devices are becoming ubiquitous, creating an excellent opportunity to utilize high throughput analytics to glean important insights and enable more precise, personal, and effective health care. Such advances are especially crucial for the most vulnerable patients with complex, chronic conditions, such as children with single ventricle heart defects.
A year ago, McElhinney moved across the country to Stanford to take the helm as director of the Children’s Heart Center’s new Clinical and Translational Research Program. The novel program, one of only a few like it in the nation, provides critical infrastructure for collaborative pediatric heart research at Lucile Packard Children’s Hospital Stanford and brings clinicians and researchers together to speed the process of translating basic science findings into medical advances.
Through these collaborations, McElhinney is keen to explore novel areas of research. “Although tremendous advances have been made in the management of children and adults with congenital heart disease, there are still many unsolved problems and opportunities to move the field forward,” he notes.
The Clinical and Translational Research Program is pursuing investigations into the fundamental pathophysiology, evaluation, and care of patients; the development of new medical devices and technologies; and the use of big data to analyze information from patient records and further our understanding of complex clinical and pathological processes.
McElhinney aims to elucidate how personalized medicine can be applied to cardiac care. “Oncologists use different drugs based on the type of mutations, and that allows for different care,” he says. “Maybe it’s the same thing in congenital heart disease. A clearer, finer understanding of that patient may allow for more fine-tuned medication.” Stem cell therapies are another arena that McElhinney is eager to investigate as a promising treatment for congenital heart defects—one that can be personalized to each patient’s needs.
Fortunately, McElhinney is in the perfect place to find answers to these important questions. The Clinical and Translational Research Program is ideally situated at Stanford University, a research-intensive institution already leading the way in using big data to tackle challenges in biomedicine and make a global impact on the way we diagnose, treat, and prevent disease.
“Stanford has a dynamic, innovative culture in terms of working with information in creative and progressive ways,” McElhinney says. “We’re hoping to leverage that culture to do unique things in the cardiac program. We’re really hitting our stride and bringing a lot of people together.”
McElhinney says that one of the draws of pediatric cardiology for him are the challenges that still need to be addressed, considering the anatomical and physiological complexities pediatric cardiologists deal with. “In so many dimensions, there’s more to learn. It’s not the same job it was when I started,” he says. “And it won’t be the same in 10 years.”
Alison Marsden, PhD
Associate Professor of Pediatrics (Cardiology) and of Bioengineering
A decade ago, Alison Marsden, PhD, was designing quieter airplane wings. But when she completed her dissertation on wing optimization for her PhD in mechanical engineering at Stanford, she knew she wanted to pursue something more down-to-earth for her postdoctoral studies.
As it turns out, her background in mechanical and aerospace engineering provided the perfect foundation for her current role—designing better surgeries for children with congenital heart disease.
“I was interested in working on something that had a more human application,” she says. “And I found it extremely motivating to work on medical problems in children.”
Partnering with Jeffrey Feinstein, MD, MPH, a pediatric cardiologist and the Dunlevie Family Professor of Pediatrics (Cardiology) at Stanford, Marsden spent long hours learning about cardiology and shadowing Feinstein on his rounds at Lucile Packard Children’s Hospital
Stanford. “He would quiz me like I was a med student,” Marsden recalls, laughing.
Eventually, Marsden and Feinstein became major collaborators.
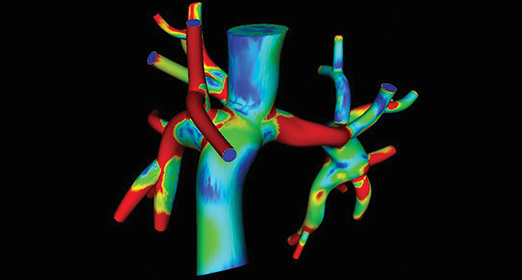
Marsden, who recently returned to Stanford as associate professor of pediatrics in cardiology after several years on the faculty at the University of California, San Diego, applies her knowledge of fluid mechanics to understand how blood flows through the hearts and blood vessels of children with congenital heart defects. She started out using her computer simulations diagnostically and now also uses computer models to find ways to improve surgical procedures.
Teams building engineering solutions to medical problems are relatively common, Feinstein explains, but ensuring those theoretical designs will work in a clinical setting, as Marsden does, is a higher hurdle to jump. “It’s a miniscule group of people who choose to do what she does, and who do it as well as she does,” Feinstein says. “Having her as a member of our pediatric cardiology faculty is a tremendous asset as we seek to further refine care for our patients.”
Marsden’s work provides a way of testing new surgery designs on the computer before trying them on patients, much like engineers use computer codes to test new designs for airplanes or automobiles. Marsden and her team have simulated a refinement of a surgical procedure called a Fontan—the last step in a series of operations that children with single ventricle defects must undergo in order to survive.
In the Fontan surgery, the large veins returning blood to the heart from the body are directly connected to the arteries that send deoxygenated blood to the lungs, forming a modified T-shaped junction. This bypasses the heart on the one side so that the resulting circulation puts the single pumping chamber to optimal use. Using models derived from MRI image data, Marsden used computer models to design and optimize a new type of connection for the Fontan procedure in the shape of a Y (called the Y-graft Fontan). Computer models can help pediatric surgeons determine whether this procedure will benefit a patient, as well as how a patient’s heart will perform during moderate exercise.
“Computer modeling makes it possible to test out high-risk, high-reward approaches,” Marsden says. “It gives us a way to test out-of-the-box ideas with no risk to patients.”
That Y-graft refinement was recently successfully tested in a pilot study in six patients with promising clinical outcomes. Marsden is now beginning to develop refinements and novel approaches to other pediatric heart surgeries as well.
“Alison’s work enables us to look at things we can’t look at in any other way,” says Feinstein. “The whole concept of simulation-based medicine offers opportunities to try things with zero risk to the patients. With this type of computer modeling, you can do 100 simulations before you ever try it in a patient.”
Sushma Reddy, MD
Assistant Professor of Pediatrics (Cardiology)
Can tiny strands of mouse RNA teach us how to help children with congenital heart defects live longer, healthier lives? Sushma Reddy, MD, assistant professor of pediatrics in cardiology, says yes, and is uncovering signals in mouse blood that can warn doctors about impending heart failure.
Reddy first became interested in cardiology as a medical student. Why cardiology? “It’s the physiology that makes it absolutely exciting and it makes you think on your feet,” Reddy says. Since then, there have been tremendous strides in treating congenital heart defects, and patients are staying alive and doing better than they were 20 years ago.
“We are continuing to push technological boundaries to aid our patients,” she says, noting that there are still many unanswered questions.
Reddy is particularly interested in children born with one functioning ventricle—a condition called single ventricle heart defect. Even after a series of complex surgeries to improve the situation, these children continue to have one pumping chamber instead of two, and they are thereby not as strong as children with normal hearts.
“At some point in time, all these children go into heart failure—all of them,” explains Reddy. Doctors use echocardiograms and cardiac MRIs to keep tabs on how well the hearts of these patients are doing, but early signs of trouble may be undetectable using these imaging techniques. By the time they show clinical evidence of heart failure, the disease is advanced and most medical therapies are ineffective in reversing this process.
Reddy is tackling this problem by studying heart defects in mice that mimic the problem human congenital heart disease patients have. Specifically, she studies the machinery that regulates the genes and proteins mediating heart failure. She found that tiny strands of RNA—microRNAs— are one marker of impending heart failure. “We’ve identified a group of microRNAs that we think are important,” says Reddy. “They give us clues of when the heart is beginning to decompensate, much sooner than the current methods that we use. We have early data in children with heart disease showing that these biomarkers are useful predictors of disease progression.”
She is currently collaborating with other centers to see if the blood biomarkers she identified in mice are also present in children on a larger scale. The best approach, she says, is to combine blood biomarker tests with current imaging tests to give the most complete picture of how well a patient with congenital heart disease is doing.
Reddy’s research recently received a major boost when she was awarded a Mentored Clinical Scientist Research Career Development Award, a K08 grant from the National Institutes of Health (NIH), which will fund her work on understanding the mechanisms of heart failure and the development of biomarkers over the next five years. In her first years at Stanford, Reddy relied on private sources such as grants from the Children’s Heart Foundation and a gift to the CVICU to fund her research. Those early grants and gift funding allowed her to establish preliminary data, which she has now successfully leveraged to garner the prestigious NIH grant. “It was the private funding that helped me get where I am now,” she notes.
Reddy says she admires the willingness of her patients to try new approaches. “The children and families I see are amazing,” she says. “They are born with the disease and live with it for their life span. It’s a privilege to take care of them, and their perseverance motivates me to find the best possible treatment.”
This article first appeared in the Fall 2015 issue of Lucile Packard Children's News.